A carboxylate is the conjugate base of a carboxylic acid. Carboxylate salts are salts that have the general formula M(RCOO)n, where M is a metal and n is 1, 2...4 years ago. Carboxylate Anion.This is a standard acid base reaction. Propanoic acid is fairly weak, yet will react with sodium hydroxide. Sodium propanoate would give a basic solution in aqueous solution.propanoic acid. The acid strength of carboxylic acid are strongly modulated by the moiety attached to the carboxyl. However, the acid-base reaction is much faster, which yields the non-electrophilic carboxylate and the non-nucleophilic ammonium, and no further reaction takes place.When a carboxyl group is deprotonated, its conjugate base forms a carboxylate anion. To more easily understand much of the below discussion of reactions involving carboxylic acids it can be helpful to notice that the carboxyl group itself is a "hydroxylated carbonyl group" meaning Propanoic acid.
Draw the resulting carboxylate anion that forms when propanoic...
Binary acids are certain molecular compounds in which hydrogen is combined with a second nonmetallic element; these acids include HF, HCl, HBr, and HI. When a carboxyl group is deprotonated, its conjugate base, a carboxylate anion, forms.Carboxylic acids are weak acids and their carboxylate ions are strong conjugate bases. 1. Esterification : When carboxylic acid reacts with alcohol in the presence of conc. H2SO4 to form Reaction with : (a) Tollen's reagents : formic acid behaves as a reducing agent and reduces Tollen's...propanoic acid. Because of their enhanced acidity, carboxylic acids react with bases to form ionic salts, as shown in the following equations. In the case of alkali metal hydroxides and simple amines (or ammonia) the resulting salts have pronounced ionic character and are usually soluble in water.Correct Part C Draw the resulting carboxylate anion that forms when propanoic acid reacts with a strong base. Draw the molecule on the canvas by choosing Correct Draw the Structure Question 46 Part A Draw the structure of the organic product of the reaction between benzoic acid and ethanol.

How would you draw the resulting carboxylate anion that forms...
Carboxylic acids react with carbonates and hydrogencarbonates to form a salt, carbon dioxide and water. The resulting bond between the sodium and the ethanoate group is ionic. It must not be represented by Propanoic acid + sodium carbonate → sodium propanoate + water + carbon dioxide.Draw the resulting carboxylate anion that forms when propanoic acid reacts with a strong Step 5 Propanoic acid is first converted to the acid chloride by treatment with SOCl2 and then reacted with the 3 4 dichloroaniline to give the desired forms when propanoic acid reacts with a strong base.lists common strong acids and strong bases, it is wise to memorize this table as this will be useful in solving titration problems. An acid that is completely ionized in aqueous solution. This means when the strong acid is placed in a solution such as water, all of the strong acid will dissociate into its ions...When a strong base reacts with propanoic acid, the only replaceable hydrogen atom in the compound is removed. Recall that carboxylic acids are often monobasic, hence the removal of that hydrogen creates a carboxylate ion whose structure has been shown in the answer section.19. 1. Carboxylic acids: reactions When a strong base is added to a carboxylic (weak) acid, water and salt are formed. Although the 33. 2. Esters: reactions The carboxylic acid that forms when the ester breaks up reacts immediately with the base, forming the carboxylate anion, associated...
Yahoo Answers is shutting down on May 4th, 2021 (Eastern Time) and the Yahoo Answers web site is now in read-only mode. There can be no adjustments to other Yahoo homes or services, or your Yahoo account. You can in finding extra details about the Yahoo Answers shutdown and download your information in this assist page.
Separation of an Unknown Mixture

Solved: Rank From Highest To Lowest Boiling Point . To Ran ...

Chemistry Archive | May 24, 2015 | Chegg.com
Chemistry Archive | April 24, 2017 | Chegg.com
Chemistry Archive | September 17, 2014 | Chegg.com

Chemistry Archive | April 24, 2017 | Chegg.com

Answered: Draw the resulting carboxylate anion… | bartleby

Solution: Circle all the Lewis Bases in t... | Organic Chem

Reactions of Carboxylic Acids - Chemistry LibreTexts
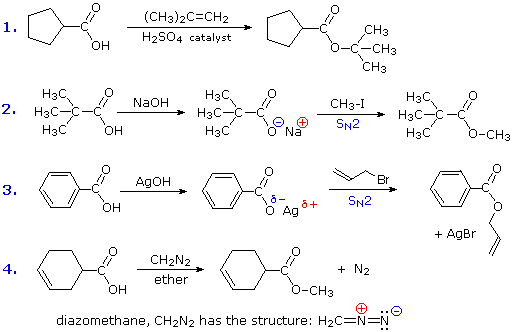
Assignment 7: Aldehydes, Ketones, Carboxylic Acids and ...

Complete this reaction of a carboxylic acid with strong ...

Chemistry Archive | May 24, 2015 | Chegg.com
Assignment 7: Aldehydes, Ketones, Carboxylic Acids and ...

Chemistry Archive | May 24, 2015 | Chegg.com
Pin on ORGO Reactions

Hint 3 Determine the name of the carboxylic acid portion ...

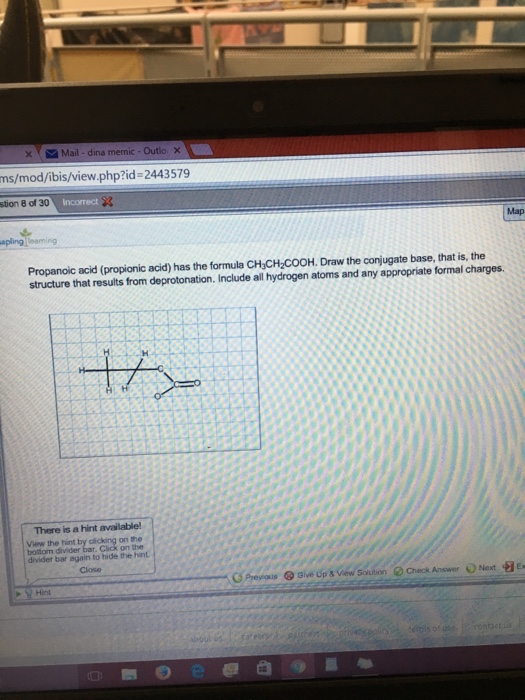
Solved: Propanoic Acid (propionic Acid) Has The Formula CH ...
£½ÕÂ 16 Section D Other Oxygen-Containing Functional Groups

Hint 3 Determine the name of the carboxylic acid portion ...

Chemistry Archive | September 17, 2014 | Chegg.com

0 comments:
Post a Comment